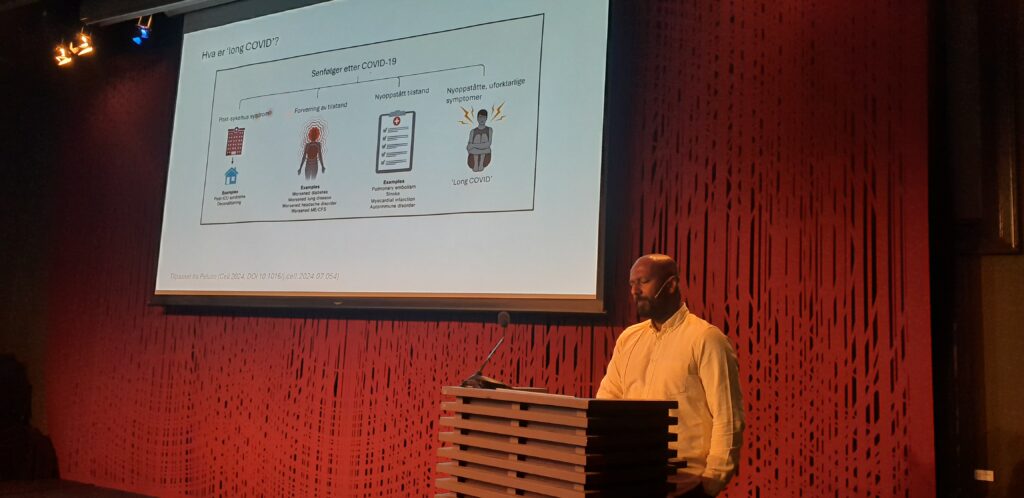
In our next NSI Rising Star Seminar, we will be hosting Klaus Eyer (PhD, associate professor, Department of Biomedicine, Aarhus University) with a talk on “The Many Functions of individual Immune Cells: Exploring Polyfunctionality and Polyspecificity on the single-B cell level”. Look forward to seeing you there!
Meeting details:
Speaker: Klaus Eyer
Title: The Many Functions of individual Immune Cells: Exploring Polyfunctionality and Polyspecificity on the single-B cell level
Time and date: Thursday, June 19 at 14:00
Meeting link: https://uio.zoom.us/j/61981540552
Talk abstract:
Immune responses are driven by various individual cells acting without central coordination. Each cell contributes distinct functions that dynamically adapt, and the collective behavior ultimately shapes the immune outcome. Indeed, single-cell analysis can advance our understanding of these responses with the necessary resolution. While single-cell analysis has advanced significantly in proteomics, genomics and transcriptomics, these approaches only partially reflect actual cellular function and activity. Therefore, our laboratory is working on developing systems to quantify, apply and use individual cellular functions in fundamental questions and clinical applications to unlock their full transformative and translational potential.
During this presentation, we will discuss two very recent, not yet published studies from our laboratory. The first focuses on polyfunctionality of individual B cells, and its temporality – the ability of cells to secrete multiple, distinct proteins either sequentially or simultaneously, and the potential impacts of temporality on function. The second focuses on a new technique that analyzes individual antibodies for their potential to be polyreactive. Indeed, current technologies with single-antibody resolution typically measure binding to only one or a few recombinant antigens, often ignoring specificity and polyreactivity as parameters. We present a method for screening antibody repertoires at single-antibody resolution against a large library of protein variants, using SARS-CoV-2 receptor-binding domain (RBD) mutants to identify the antigenic spectrum of rare, polyreactive antibodies. Using multiplexed antigen sequencing, we performed mutational scanning, enrichment, and escape analysis to map epitopes of the sum and individual polyreactive antibodies within the immunization-generated antibody repertoire.
More information about Klaus Eyer:
Dr. Klaus Eyer has been an associate professor at the Department of Biomedicine at Aarhus University since February 2024. He has a background in pharmaceutical sciences and earned his doctorate in Bioanalytics from ETH Zurich, and his research focuses on functional immune repertoire analysis and single-cell technologies. In the past, Dr. Eyer has held academic positions at ETH Zurich in Switzerland and ESPCI Paris in France and combines different disciplines in his work.
He collaborates locally and internationally on projects related to antibody discovery and immune profiling/characterization, with a focus on their application in neuroinflammation, rare diseases, and personalized medicine. In addition to his research, he is actively involved in teaching, mentoring, and translational activities, including co-founding a biotech start-up, coordinating a doctoral network throughout Europe and contributing to public discussions on science and adaptive immunity following immunization.
Google scholar: https://scholar.google.fr/citations?user=PRtGoGAAAAAJ&hl=en
Key papers:
1. Single-cell deep phenotyping of cytokine release unmasks stimulation-specific biological signatures and distinct secretion dynamics
2. Single-cell deep phenotyping of IgG-secreting cells for high-resolution immune monitoring